We speak of oven temperature as the main variable that determines how fast a cake will bake or any food will cook. But although the temperature is of primary importance, it is only one factor. Even at exactly the same oven temperature, the amount of heat energy a food actually receives and absorbs may not be the same.
By “oven temperature,” we mean the temperature of the air inside the enclosure, and that’s what the temperature control device regulates. But once the air is heated to a certain temperature, there are still three ways in which the air’s heat can be transmitted into the food: by conduction, by convection, and by radiation.
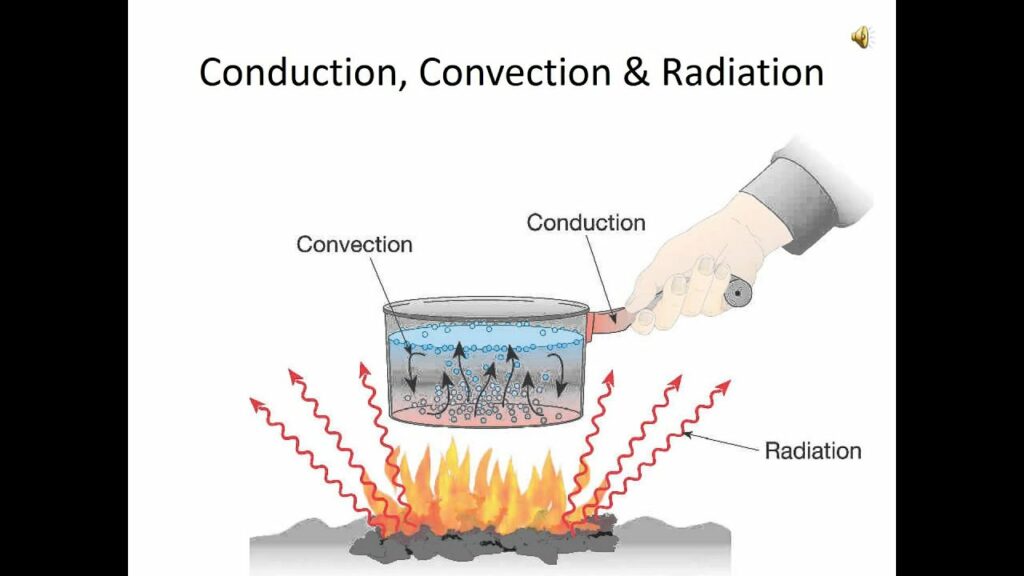
Conduction
When two substances at different temperatures are in contact, such as hot oven air in contact with a food’s surface, heat will flow from the higher-temperature air into the lower-temperature food by the process of conduction. Just as water always flows downhill if it can, heat will always try to flow “down-temperature” from high to low. The heat energy is conducted from the air to the food across their interface by direct molecular collisions. That is, the hot air molecules are moving faster than the cooler food molecules (that’s actually the definition of temperature: it’s the average motion energy, kinetic energy, of the molecules), and when they collide with the food’s molecules they kick them up to a faster (hotter) speed, like a cue ball scattering a rack of billiard balls.
But conduction is very inefficient. Air molecules are separated from one another by interplanetary distances, relatively speaking, so the chances that a hot air molecule will collide with the surface of a cake pan or a roast are small. Heat conduction can be quite efficient between two solids in contact, such as your hand on a hot frying-pan handle, but not between hot air and anything else. You can put your hand in a 200°F (93°C) oven for several seconds without fear, because the rate of conduction of heat from the air into your skin is so extraordinarily slow. But don’t try dipping your hand into 200°F water. Water is a much better conductor of heat because its molecules are much closer together than air’s are.
And why are metals the best heat conductors of all?
In almost all other materials, the atomic electrons are parts of individual molecules. But in metals, the electrons belong, in effect, to all the atoms simultaneously. We can think of metal atoms as being embedded in a swarm or sea of shared electrons, like raisins in an electron pudding. When a metal comes in contact with the agitated molecules of a hot substance, it’s the electron swarm that transfers the agitation, the heat, rapidly to all other parts of the metal. That’s heat conduction.
In an oven, however, the other two heat transmission mechanisms, convection and radiation, are more important than conduction.
Convection
Variable conditions inside the oven, such as inevitably uneven temperatures between one spot and another, make the air move, because hotter “pieces” of air rise, while cooler “pieces” fall, creating a kind of circulation that’s called convection, or convection currents. This circulation boosts the efficiency of heat transfer between the air and the food, because it increases the amount of contact between the food and the hot air molecules in the enclosure. A convection oven enhances this effect by means of a fan that circulates the oven’s internal air or some externally heated and blown-in hot air, leading to more efficient heat transfer and faster cooking. That’s why it’s good practice to lower the temperature by about 25°F (14°C) when using a convection oven rather than a standard one.
Radiation
The third mechanism by which food becomes hot in an oven is by absorbing radiation. The oven’s heating element or flame and its walls and floor are hot, they are what make the air hot, and hot things radiate infrared radiation. In fact, all materials at all temperatures are emitting some of their energy as infrared radiation.
For a given object, the hotter it is, the more infrared radiation it is emitting. When the infrared radiation coming from the hot oven walls and the hot air hits the food, the food molecules absorb it and move with increased energy. That is, they become hotter.
Infrared radiation isn’t heat, as many books will tell you. It is electromagnetic radiation, like radio, radar, and microwaves, but of just the right wavelength to be absorbed by most kinds of molecules, which thereby become more energetic and hotter. I call infrared radiation “heat in transit,” because it is emitted by hot matter and travels through space, yet it isn’t transformed back into heat until it is absorbed by other matter.
